- Gut health and immune function are central to preventing illnesses that negatively impact athletic performance. More recently, evidence suggests that gut health and immune function may play a role in promoting adaptation to exercise.
- Gut bacteria, or the microbiota, perform several vital functions, including regulating mucosal immune activity, modulating host metabolic activity, producing short chain fatty acids, enzymes and vitamins, and protecting against intestinal infection.
- Dietary manipulation may enhance gut bacteria composition and metabolic activity and promote optimum immune function.
- Probiotics have been the primary focus of research into modulating gut bacteria, with some studies showing that supplementation reduces gastrointestinal and upper respiratory symptoms and modulates some aspects of the immune system.
- Prebiotics, synbiotics and isolated peptides from whey and colostrum require further research before practical recommendations can be developed.
- Athletes are advised to work with their dieticians to modify their diet and determine whether supplements, such as probiotics and prebiotics, may be useful during prolonged exercise, periods of heavy training, and during competition and travel.
INTRODUCTION
There is a strong focus on gut health and immune function by athletes, given evidence that the intestinal microbiota can positively influence the immune system and reduce the risk of illness. The intestinal microbiota comprises only one aspect of gut health and needs to be considered alongside intestinal permeability and the mucosal immune system. It is through the mucosal immune system of the gut that the intestinal microbiota influences immune activity at other sites of the body, including the upper respiratory tract. Improving immune function in the respiratory tract is of high importance to athletes and their coaches given that upper respiratory symptoms (URS) remain the most common illnesses experienced by athletes, particularly those involved in prolonged, intense exercise and during competitions (Drew et al., 2017). A growing understanding of the interaction between gut health and metabolism suggests that the microbiota may also play an important role in adaptation to training and to performance. One hypothesis is that gut health through the microbiota contributes to sporting performance via effects on the immune system and nutrient metabolism (Figure 1). This suggests that a healthy gut may be central to optimum sporting performance.
Understanding of the role of the microbiota has driven interest in the use of gut health supplements, in particular probiotics, and to a lesser extent, prebiotics, synbiotics and immune proteins isolated from whey. Traditionally, probiotics have been defined as microorganisms that when ingested at sufficient doses provide a health benefit to the host while prebiotics are substrates, such as non-digestible carbohydrates, that are selectively utilized by host microorganisms that confer a health benefit (Gibson et al., 2017; Hill et al., 2014). Synbiotics are supplements that combine pro- and prebiotics and that may also include other factors, such as vitamins, minerals or whey-derived proteins. Studies of sub-elite and recreational athletes are providing evidence that some probiotics reduce gastrointestinal (GI) symptoms and URS, and pos-itively alter the immune system (West et al., 2011; 2014; Michalickova et al., 2016). However, there is too little research in athlete populations to make recommendations for prebiotics, herbal supplements and flavonoids. Dietary modification, and in particular starch intake, needs to be considered for improving gut health. Athletes wishing to remain healthy should work with a dietitian to alter their diet and then to trial a supplement that is backed by research evidence. This Sports Science Exchange article will examine the role of the gut and immune system in reducing susceptibility to illness in athletes, review nutrition strategies for gut health and immune function, and consider gaps within the field.
GUT HEALTH, THE MICROBIOTA AND IMMUNE FUNCTION
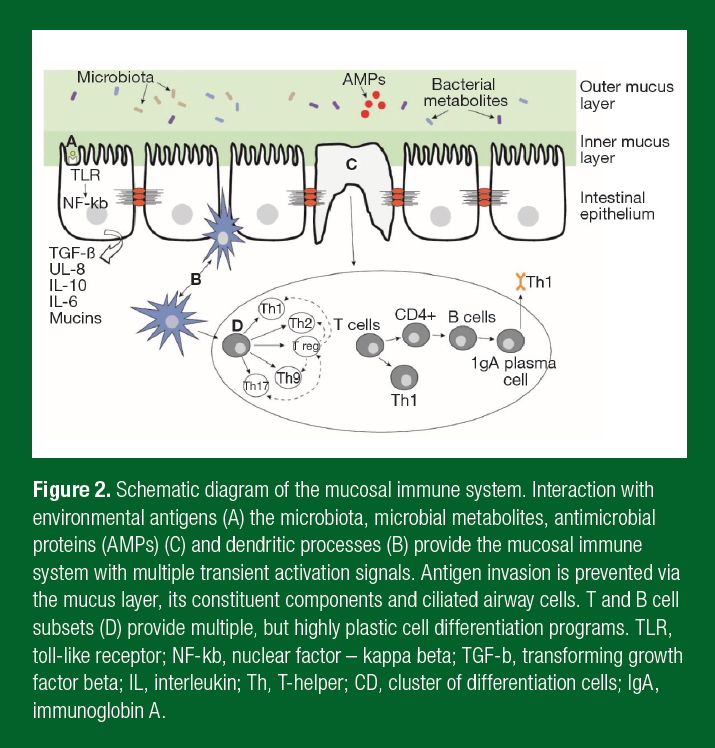
The gut plays a crucial role in the body through digestion, spatial separation of, and interaction with, commensal bacteria and protection of the body from pathogens. Gut health has been a focus in athletic research given that heavy exercise can result in gut symptoms, including diarrhea, cramps and bloating (Davison et al., 2016b). Initially this work focused on intestinal permeability, an increase in the rate of molecules crossing the intestinal epithelium. Over the last decade, focus has turned to the gut microbiota, otherwise defined as the microorganisms that inhabit the intestines. The intestinal microbiota now number ~1013 and reach their highest density in the colon. The microbiota contributes to all aspects of gut health, from the digestion of starches, the production of metabolites, the development and regulation of the
immune system, and exclusion of pathogens from infecting the body (Figure 2). Evidence strongly indicates that the microbiota also regulates gut permeability, highlighting the intricate connections that occur in the gut to maintain health (Biesalski et al., 2016). Gut bacteria exist on a spectrum of symbiosis to dysbiosis and have been implicated in diseases of the intestine, such as irritable bowel disease, and to extra-intestinal diseases including cancer, obesity and mental health (Selber- Hnatiw et al., 2017). The broad role of gut bacteria in physiology and in health has led to it being considered as an organ in its own right.
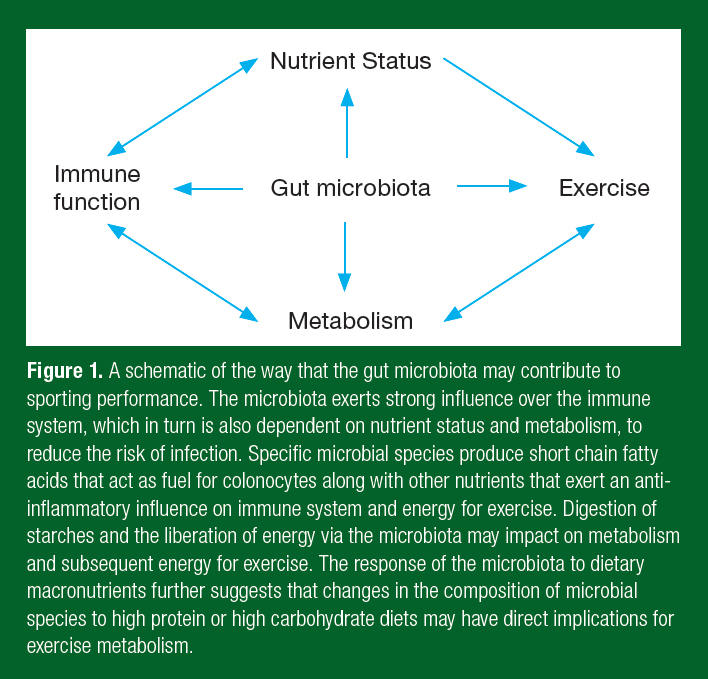
Interest in the microbiome within athletic groups is largely focused on its ability to modulate the immune system to prevent illness, particularly URS. There is strong evidence that the microbiome shapes the intestinal mucosal immune system (Kim & Kim, 2017). Microbial interaction with B- and T-cells regulates inflammatory responses and the induction of secretory immunoglobulin A (SIgA) with these microbes directly induce the secretion of antimicrobial proteins, such as the beta-defensins, and mucous. It is the regulation of B- and T-cells in the gut and subsequent trafficking to other mucosal sites, such as the respiratory tract, that provides the means for the microbiota to alter the risk of respiratory illness. Evidence strongly implicates the host microbiota in maintaining gut and respiratory health by regulating immune function.
Microbial-immune interaction may also influence exercise-induced inflammatory processes, potentially impacting on recovery and adaptation. Recovery strategies post-exercise are common and are designed to reduce inflammation and promote regeneration. Post-exercise inflammatory processes are now recognized as integral to muscle repair, regeneration and adaptation to exercise. Specific microbes and bacterial metabolites can skew inflammatory profiles toward an inflammatory or anti-inflammatory phenotype. For instance, a recent study found that Akkermansia muciniphila modified intracellular signalling pathways to moderate the stress-induced inflammatory response (Zhao et al., 2017). Furthermore, microbe growth and activity are altered in response to mammalian hormones (Lyte, 2016). Alongside evidence that exercise induces changes in the composition of the microbiota (Bressa et al., 2017), this information suggests a direct link between exercise and the composition of the microbiome and to microbes potentially influencing regulation of post-exercise immune responses. The acute and chronic response to exercise is well characterized, with the magnitude of these responses dependent on the intensity and duration of exercise. Whether specific microbial species or the composition of the microbiome influence exercise-induced changes to the immune system and their time course in the post-exercise period is an intriguing question.
THE MICROBIOTA, NUTRIENT METABOLISM AND EXERCISE
The role of the diet-microbiota-metabolism axis in obesity and metabolic syndrome provides a novel glimpse into a further role for gut bacteria in athletic performance. There is strong evidence that the microbiota contributes to energy harvest from food. Human studies of twins provide evidence of a role for the microbiota and gut health in metabolism and body composition. A study of 54 adult female monozygotic and dizygotic twin pairs concordant for body composition also reported significantly reduced intestinal bacterial diversity, a lower relative abundance of Bacteriodetes, and higher relative abundance of Actinobacteria in obese compared with lean individuals, but no significant difference in Firmicutes (Turnbaugh et al., 2009). The implications of these studies for athletic performance are profound. Greater energy harvest from food for use as a substrate, particularly in endurance athletes, may slow or prevent the depletion of energy stores that occurs during exercise. The higher availability of glucose may lower perceived exertion of effort and maintain immune function during exhaustive exercise (Qin et al., 2017). Greater energy harvest may also underpin the resistance to dietary restriction of body composition in weight-restricted athletes.
The gut microbiota may also impact host metabolism through the synthesis of a variety of vitamins, including vitamin A, vitamin B12, the active form of vitamin B6, vitamin B5, vitamin B3, biotin and vitamin K. Micronutrient intake is essential for health and metabolism, and research indicates many athletes do not meet micronutrient recom-mendations, particularly given there may be a need for higher intake with endurance exercise and exercise loads at the elite level (Wardenaar et al., 2017). There is also concern that with the availability of supplements and fortified foods that some athletes may exceed micronutrient recommendations. The effects of vitamins synthesized by the microbiota on vitamin status is an emerging area of research focus (Biesalski, 2016). Deficiencies in micronutrients adversely impact immune function and susceptibility to illness, and may compromise athletic performance. Determining the role of the microbiota on vitamin status would allow for targeted personalized intervention.
DIET, GUT HEALTH AND IMMUNE FUNCTION
Diet has a strong influence on gut health via effects on the microbiota, immune system and gut permeability. Changes in diet have a profound impact on the composition and function of the microbiota. In humans, the effect of food on the composition of the microbiota has been shown in a crossover study of 11 individuals consuming either a plant-based or animal-based diet for five days, after which the composition of their gut microbiome substantially changed to reflect either carbohydrate or protein (David et al., 2014). Animal studies clearly show that high fat diets have a strong impact on the gut microbiome that promote adiposity (Turnbaugh, 2017). There are few studies that focus on high fat feeding in humans but implementing a “healthy” diet in obese individuals is associated with altered gut bacterial profiles and improvement in metabolic indices (Haro et al., 2017). Whether the effects of high fat diets relate to the higher consumption of fat or to the restriction of starch-based carbohydrates that typically occurs in these diets is relevant to consider. The rapid response of the microbiota to dietary changes reflects changes in the availability of nutrients that different bacterial species have for survival. Changes in diet, particularly in grains and fiber, also lead to alterations in bacterial by-products, such as short chain fatty acids, that promote mucosal immune homeostasis and improve intestinal epithelial integrity. Diet is proposed to be the primary factor in determining gut microbiota diversity, which thus far is consistently reduced in diseased compared to healthy populations. Evidence for the role of diet in modification of the microbiota and gut health provides athletes (and non-athletes) with important information before they consider turning to supplements.
Carbohydrates and the Immune System
Modifying macronutrient intake is a common strategy for athletes, particularly in relation to carbohydrates. Carbohydrate intake prior to and during exercise is recognized to blunt exercise-induced inflammatory responses, with some evidence of a shift toward a T-helper (Th)-1 cell mediated immune profile. Pre-exercise carbohydrate supplementation also blunts cortisol responses and leucocyte trafficking responses in the post-exercise period. Interestingly, while carbohydrates moderate exercise-induced immune responses, carbohydrate supplementation has failed to show an effect on functional immune tests, such as a hypersensitivity challenge, in the post-exercise period (Davison et al., 2016a). In general, research supports the use of carbohydrate ingestion prior to and during strenuous exercise to blunt the post-exercise immune response.
Probiotics, Gut Health and Immune Function
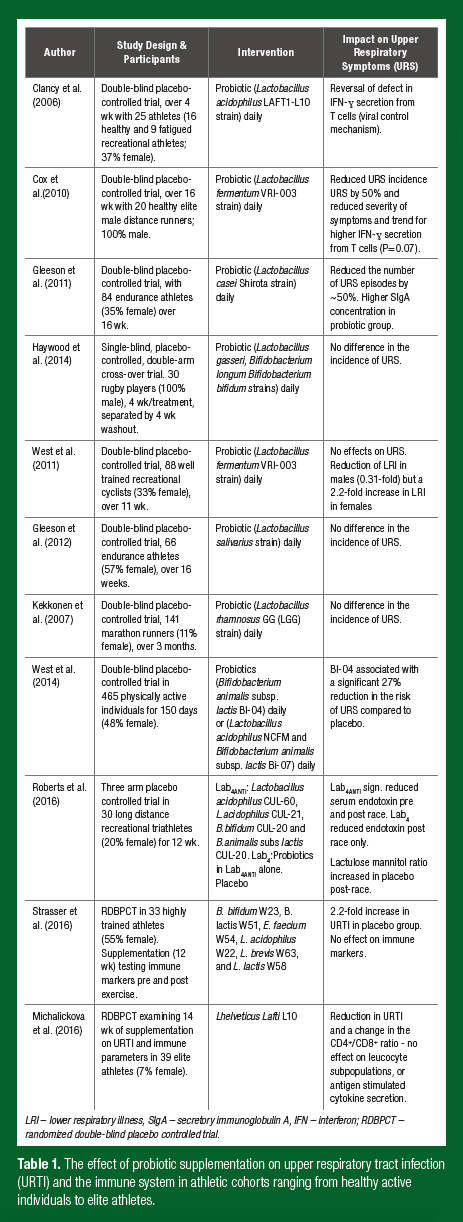
There are a range of nutrition supplement strategies employed by athletes for gut health and immune function. Of these, probiotic supplements are among the most popular. Probiotics have an established history of use for preventing GI illness, particularly traveller’s diarrhea and antibiotic associated diarrhea. Other research has focused on the effects of probiotics on immune function in athletes while examining whether probiotics reduce upper respiratory tract illness (URTI). While promising results have been observed in relation to URTI (Table 1), the changes observed in immune activity are mixed. A primary reason for this may be that most probiotic studies have examined systemic (blood) immune markers rather than respiratory markers. For instance, research by our group observed a significant 27% reduction in the risk of URTI over five months of supplementation with Bifido-bacterium animalis subspecies lactis Bl-04 (Bl-04) in 465 healthy active individuals, but no effect on resting serum cytokines, peripheral blood cluster of differentiation (CD)4+ T cell subsets, natural killer (NK)- cell function or phagocytosis (West et al., 2014). Observations of changes in local immune parameters in the respiratory tract may provide new insight into the mechanisms of probiotic supplements.
Differences in the effectiveness of probiotic supplements relate to the type of sport (endurance vs. team sport), the training history of the athlete, the training load being undertaken, along with supplement-specific differences in the strain(s), method of delivery and duration of supplementation. Research in our group has also observed gender differences in the effect of probiotic supplements on URS. In a study of 99 competitive cyclists (64 males and 35 females; age 35 ± 9 and 36 ± 9 yrs), lower respiratory symptoms were lower by a factor 0.31 (99% confidence interval (CI); 0.07 to 0.96) in males but increased by a factor of 2.2 (0.41 to 27) in females during 11 weeks of supplementation with lactobacillus fermentum (PCC) (West et al., 2011). There is now a body of research in male and female highly physically active groups to support the use of probiotics during heavy training periods, travel, and in the lead-up to and during competition (Table 1).
Prebiotics
Prebiotics are non-digestible polysaccharides that stimulate the growth and activity of the gut microbiota. A number of foods have a prebiotic effect, including chickpeas, lentils, barley, bananas, oats, wheat, soy-beans, asparagus, leek, chicory, garlic, artichoke and onion. In general, dosages above 2.5 grams are necessary to alter the abundance of microbial species, which is far higher than naturally occurs in any single food. The fermentation of these starches by GI bacteria releases metabolic by-products, including short chain fatty acids (butyrate, propionate and acetate), vitamins and lipid metabolites that modulate various aspects of the immune system and host metabolism.
Few studies have examined supplementation with prebiotics in athletes. Research by our group examined the consumption of butyrylated high amylose maize starch at a dosage of 40 g daily, mixed with a protein drink in 40 endurance trained athletes. We observed significant increases in two microbial species, Parabacteroides distasonis (81-fold (28- to 237-fold) P < 0.01) and Faecalibacterium prausnitzii (5.1-fold (2.1- to 12-fold) P < 0.01) along with significant increases in fecal butyrate and propionate and in the serum cytokines tumour necrosis factor-α and interleukin-10 (West et al., 2013). P. distasonis and F. prausnitzii have both been identified to exert an anti-inflammatory influence on the immune system and when low in diversity to be associated with compromised gut health. Interestingly, many of the athletes in this study noted improved gut health and bowel function during supplementation. Other research has combined prebiotics with probiotics. Supplementation for 12 weeks with a combined prebiotic and probiotic supplement significantly reduced markers of intestinal permeability after a long-distance triathlon in recreational athletes compared to both a probiotic or placebo supplement (Roberts et al., 2016). While research in settings outside of exercise suggests prebiotics have strong effects on the gut microbiota and on the metabolic by-products of bacterial fermentation, more research is required in athletes to identify starch-specific microbial and immune effects, dosages and durations of supplementation before practical recommendations can be proposed.
Bovine Colostrum
Bovine colostrum (BC) has been investigated in elite and recreational athletes with mixed findings. In a study of healthy male runners, eight weeks of bovine colostrum was associated with a significant increase in intestinal permeability (Buckley et al., 2009). Compared to placebo, supplementation for four weeks with 20 g of BC in well-trained cyclists was associated with a significantly greater oxidative burst capacity but no effect on leucocyte trafficking, neutrophil degranulation or the concentration of salivary IgA, lactoferrin or lysozymes (Jones et al., 2014). In contrast to these results, a study by Shing et al. (2007) found that five weeks of BC supplementation in 29 highly trained road cyclists during a high-intensity training period led to a significant increase in in pre-exercise serum soluble tumour necrosis factor receptor 1 and moderated the post-exercise decrease in cytotoxicity/suppressor T cells compared to placebo. Based on these studies it would be inappropriate to make specific recommendations regarding BC supplementation for gut health and immune function. It should be recognized that BC contains a range of other growth factors, including insulin growth factor-1 and antimicrobial proteins that may confer other health benefits. Further examinations of BC bioactive proteins and BC in prolonged intense exercise and different athletic cohorts is necessary.
Other Supplements
Vitamin D, zinc, anti-oxidants, herbals (ginseng, curcumin) and flavanols (quercetin) are other supplements that have been investigated for their immunomodulatory effects in athletes and physically active populations. Vitamin D has received extensive coverage due to evidence of vitamin D deficiency across many populations, including athletes. Vitamin D plays a central role in many biological processes, including modulation of the immune system. There is some controversy regarding the assessment of vitamin D status, with some arguing that the biologically inactive 25[OH]D is not the best measure. A recent study in eight active individuals supplementing with either zinc or zinc and colostrum reported an amelioration of exercise-induced changes in gut perm-eability (Davison 2016b). Further studies with larger sample sizes are required before specific recommendations can be proposed. Adequate vitamin and mineral intake is necessary to support health and immune function and routine testing to determine vitamin status may be necessary in athletes. Given high dose vitamin supplementation may be detrimental, athletes are advised to consult sports dieticians before deciding to supplement with vitamin and minerals. While some studies show changes in selected post-exercise immune markers with anti-oxidant, herbal and flavanol supplementation, these supplements have not been adequately investigated in the scientific literature. A recent meta-analysis of flavonoids in healthy adults (not specifically athletes) indicates that flavonoid supplementation decreases URTI, but has only trivial effects on a range of serum immune markers (Somerville et al., 2016). Further research to determine specific gut health and immune effects, with regards to dosage, athlete population cohort (endurance vs. team sport; recreational vs. elite athlete) is necessary before guide-lines can be developed.
FOOD FOR THOUGHT
With the continued evolution of high throughput sequencing and new technology, along with big data approaches, we are gaining greater insight into the gut, particularly the microbiota, and the immune system. The “-omics” approaches are allowing investigation of the structure-function relationship between specific microbial species and their biological roles. It is clear, that to tailor interventions for gut health and the immune system in athletes, we need a greater understanding of the microbiota in athletes. Questions include whether there are sport-specific differences, changes with training load and exercise type, gender differences and how nutrition interventions all affect the microbiota. Importantly, there is a need to better understand microbe-related metabolite production, and in particular, short chain fatty acids. Digital gene expression profiling and mass spectrometry is providing ever greater resolution to undertake quantitative phenotyping of the immune system. Given the compartmentalization of the immune system, more focus on the mucosal surfaces, particularly the respiratory tract, may yield a better understanding of maintaining homeostasis and identifying targets to prevent illness for optimum performance.
PRACTICAL APPLICATIONS
- Dietary modification should be addressed to improve gut health and immune function before supplementation is considered, particularly in relation to increasing the diversity of the microbiota with dietary fibers.
- Some probiotic supplements have an evidence base for reducing URS and GI symptoms in athletic cohorts. Dosages in commercial applications are consistent with the dosage used in research trials. Consuming greater amounts than recommended should be approached with caution and trialled before travel and competition.
- Carbohydrate intake pre-exercise and during exercise moderates the exercise-induced immune response, which may be important during heavy training periods or prolonged exercise.
- Adequate energy intake is coming to the fore as a strong predictor of greater susceptibility to illness and impaired immune function. Meeting the energy demands of exercise is important for good health.
SUMMARY
Gut health and immune function have traditionally been a focus for athletes to prevent illnesses that negatively affect performance. The gut microbiota performs several vital functions, including regulating mucosal immune activity, modulating host metabolic activity, producing short chain fatty acids, enzymes and vitamins, and providing protection from intestinal infection. The microbiota and immune system may also influence adaptation to training and performance via effects on metabolism and nutrient status. Evidence strongly indicates that diet rapidly modifies the microbiota, while carbohydrates pre-exercise and during exercise moderate exercise-induced immune responses. Some commercially available probiotic supplements now have an evidence base of benefit for upper respiratory symptoms and gut health. Other supplements, including prebiotics, colostrum and herbal supplements, require further research before practical recommendations can be proposed to athletes.
REFERENCES
Biesalski, H.K. (2016). Nutrition meets the microbiome: micronutrients and the microbiota. Ann. NYAS. 1372:53-64.
Bressa, C., M. Bailén-Andrino, J. Pérez-Santiago, R. González-Soltero, M. Pérez, M. G. Montalvo- Lominchar, J.L. Maté-Muñoz, R. Domínguez, D. Moreno, and M. Larrosa (2017). Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One 12: e0171352.
Buckley, J.D., R.N. Butler, E. Southcott, and G.D. Brinkworth (2009). Bovine colostrum supplementation during running training increases intestinal permeability. Nutrients 1:224- 234.
Clancy, R.L., M. Gleeson, A. Cox, R. Callister, M. Dorrington, C. D'Este, G. Pang, D. Pyne, P. Fricker, and A. Henriksson (2006). Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus. Br. J. Sports Med. 40:351-354.
Cox, A.J., D.B. Pyne, P.U. Saunders, and P.A. Fricker (2010). Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. Sports Med. 44:222-226.
David, L.A., C.F. Maurice, R.N. Carmody, D.B. Gootenberg, J.E. Button, B.E. Wolfe, A.V. Ling, A.S. Devlin, Y. Varma, and M.A. Fischbach (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559-563.
Davison, G., C. Kehaya, B.C. Diment, and N.P. Walsh (2016a). Carbohydrate supplementation does not blunt the prolonged exercise-induced reduction of in vivo immunity. Eur.J. Nutr. 55:1583- 1593.
Davison, G., T. Marchbank, D.S. March, R. Thatcher, and R.J. Playford (2016b). Zinc carnosine works with bovine colostrum in truncating heavy exercise–induced increase in gut permeability in healthy volunteers. Am. J. Clin. Nutr. 104: 526-536.
Drew, M.K., N. Vlahovich, D. Hughes, R. Appaneal, K. Peterson, L. Burke, B. Lundy, M. Toomey, D. Watts, and G. Lovell (2017). A multifactorial evaluation of illness risk factors in athletes preparing for the Summer Olympic Games. J. Sci. Med. Sport. E-pub ahead of print (PMID: 28954799).
Gibson, G.R., R. Hutkins, M.E. Sanders, S.L. Prescott, R.A. Reimer, S.J. Salminen, K. Scott, C. Stanton, K.S. Swanson, P.D. Cani, K. Verbeke, and G. Reid. (2017). The International Scientific Association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14:491-502.
Gleeson, M., N.C. Bishop, M. Oliveira, and P. Tauler (2011). Daily probiotic's (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int. J. Sport Nut.r Exerc. Metab. 21:55- 64.
Gleeson, M., N.C. Bishop, M. Oliveira, T. McCauley, P. Tauler, and C. Lawrence (2012). Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int. J. Sport Nutr. Exerc. Metab. 22:235-242.
Haro, C., S. García-Carpintero, O.A. Rangel-Zúñiga, J.F. Alcalá-Díaz, B.B. Landa, J.C. Clemente, P. Pérez-Martínez, J. López-Miranda, F. Pérez-Jiménez, and A. Camargo (2017). consumption of two healthy dietary patterns restored microbiota dysbiosis in obese patients with metabolic dysfunction. Mol. Nutr. Food Res. 2017 Sep 20. [Epub ahead of print]
Haywood, B.A., K.E. Black, D. Baker D, J. McGarvey, P. Healey, and R.C. Brown (2014). Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. J. Sci. Med. Sport 17:356-360.
Hill, C., F. Guarner, G. Reid, G.R. Gibson, D.J. Merenstein, B. Pot, L. Morelli, R.B. Canani, H.J. Flint, S. Salminen, P.C. Calder, and M.E. Sanders (2014). Expert consensus document: The International Scientific Association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11:506- 514.
Jones, A.W., S.J. Cameron, R. Thatcher, M.S. Beecroft, L.A. Mur, and G. Davison (2014). Effects of bovine colostrum supplementation on upper respiratory illness in active males. Brain Behav. Immun. 39:194-203.
Kekkonen, R.A., T.J. Vasankari, T. Vuorimaa, T. Haahtela, I. Julkunen, and R. Korpela (2007). The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int. J. Sport Nutr. Exerc. Metab. 17:352-363.
Kim, M., and C.H. Kim (2017)."Regulation of humoral immunity by gut microbial products. Gut Microbes. 8:392-399.
Lyte, M. (2016). Microbial endocrinology in the pathogenesis of infectious disease." Microbiol. Spectr. 4:2.
Michalickova, D., R. Minic, N. Dikic, M. Andjelkovic, M. Kostic-Vucicevic, T. Stojmenovic, I. Nikolic, and B. Djordjevic (2016). Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: a randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 41:782-789.
Qin, L., S. Wong, F.-H. Sun, Y. Huang, S. Sheridan, and C. Sit (2017). The effect of carbohydrate and protein co-ingestion on energy substrate metabolism, sense of effort, and affective responses during prolonged strenuous endurance exercise. Physiol. Behav. 174:170-177.
Roberts, J.D., C.A. Suckling, G.Y. Peedle, J.A. Murphy, T.G. Dawkins, and M. . Roberts (2016). An exploratory investigation of endotoxin levels in novice long distance triathletes, and the effects of a multi-strain probiotic/prebiotic, antioxidant intervention. Nutrients 8:733.
Selber-Hnatiw, S., et al. (~100 authors) (2017) Human Gut Microbiota: Toward an Ecology of Disease.
Front. Microbiol. 8:1265.
Shing, C.M., J. Peake, K. Suzuki, M. Okutsu, R. Pereira, L. Stevenson, D.G. Jenkins, and J.S. Coombes (2007). Effects of bovine colostrum supplementation on immune variables in highly trained cyclists. J. Appl. Physiol. 102:1113-1122.
Somerville, V.S., A.J. Braakhuis, and W.G. Hopkins (2016). Effect of flavonoids on upper respiratory tract infections and immune function: a systematic review and meta-analysis. Adv. Nutr. 7:488-497.
Strasser, B., D. Geiger, M. Schauer, J. M. Gostner, H. Gatterer, M. Burtscher, and D. Fuchs (2016). Probiotic supplements beneficially affect tryptophan–kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: a randomized, double-blinded, placebo-controlled trial. Nutrients 8:752.
Turnbaugh, P.J., M. Hamady, T. Yatsunenko, B.L. Cantarel, A. Duncan, R.E. Ley, M.L. Sogin, W.J. Jones, B.A. Roe, J.P. Affourtit, M. Egholm, B. Henrissat, A.C. Heath, R. Knight, and J.I. Gordon (2009). A core gut microbiome in obese and lean twins. Nature 457:480-484.
Turnbaugh, P.J. (2017). Microbes and diet-induced obesity: Fast, cheap and out of control. Cell, Host Microbe 21:278-281.
Wardenaar, F., N. Brinkmans, I. Ceelen, B. Van Rooij, M. Mensink, R. Witkamp, and J. De Vries (2017). Micronutrient intakes in 553 dutch elite and sub-elite athletes: prevalence of low and high intakes in users and non-users of nutritional supplements. Nutrients 9:142.
West, N.P., D.B. Pyne, A.W. Cripps, W.G. Hopkins, D.C. Eskesen, A. Jairath, C.T. Christophersen, M.A. Conlon, and P.A. Fricker (2011). Lactobacillus fermentum (PCC(R)) supplementation and gastrointestinal and respiratory-tract illness symptoms: a randomised control trial in athletes. Nutr. J. 10:30.
West, N.P., C.T. Christophersen, D.B. Pyne, A.W. Cripps, M.A. Conlon, D.L. Topping, S. Kang, C.S. McSweeney, P.A. Fricker, D. Aguirre, and J. M. Clarke (2013). Butyrylated starch increases colonic butyrate concentration but has limited effects on immunity in healthy physically active individuals. Exerc. Immunol. Rev. 19:102-119.
West, N.P., P.L. Horn, D.B. Pyne, V.J. Gebski, S.J. Lahtinen, P.A. Fricker, and A.W. Cripps (2014). Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin. Nutr. 33:581-587.
Zhao, S., W. Liu, J. Wang, J. Shi, Y. Sun, W. Wang, G. Ning, R. Liu, and J. Hong (2017). Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J. Mol. Endocrinol. 58:1-14.